

Release
Savara Reports Fourth Quarter / Year-End 2019 Financial Results and Provides Business Update
- Announced Positive Results from IMPALA Open-Label Follow-Up Period That Demonstrate Continued Improvement After Longer Term Exposure to Molgradex
- Company Planning an Additional Phase 3 Study of Molgradex for the Treatment of Autoimmune Pulmonary Alveolar Proteinosis (aPAP)
- Announced Top Line Microbiology Data from OPTIMA, a Phase 2a Clinical Study in Nontuberculous Mycobacterial (NTM) Lung Infection
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200312005455/en/
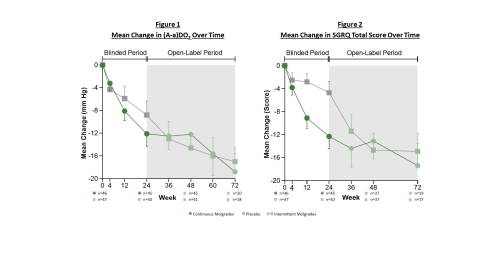
Figures 1 and 2 (Graphic: Business Wire)
“Results from the open-label period of IMPALA reaffirm our strong belief that Molgradex could improve outcomes for patients with aPAP, a rare and debilitating lung disease,” said
PROGRAM UPDATES
Molgradex for aPAP
-
Announced the
U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy Designation for Molgradex, an inhaled formulation of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), for the treatment of aPAP. - Working in consultation with the FDA to plan a second Phase 3 study.
- Completed enrollment in IMPALA-X, an open-label multicenter extension study to determine the long-term safety and use of Molgradex in patients with aPAP. Sixty out of 64 eligible patients enrolled in the study.
The Company today announced results from the open-label follow-up period of IMPALA, a Phase 3 study that evaluated Molgradex for the treatment of aPAP. The results, found below, will be discussed on today’s conference call/webcast. Data from the double-blind period of IMPALA were presented at the 2019
IMPALA Study Design
IMPALA was a randomized, double-blind, placebo-controlled clinical study designed to compare the efficacy and safety of Molgradex with placebo in patients with aPAP. Patients were randomized to receive treatment for 24 weeks in one of three arms: 1) Molgradex 300 µg administered once daily continuously over 24 weeks, 2) Molgradex 300 µg, and matching placebo, each administered once daily every other week, or 3) inhaled placebo administered once daily continuously over 24 weeks. At the end of the 24-week double-blind period, all treatment arms rolled into a 24-week open-label follow-up period and received Molgradex 300 µg administered once daily every other week.
The primary endpoint was change from baseline in alveolar-arterial oxygen gradient, or (A-a)DO2, to week 24. In addition, three key secondary endpoints―St. George’s Respiratory Questionnaire (SGRQ), six-minute walk distance (6MWD), and time to requirement for whole lung lavage (WLL)―along with multiple other secondary and exploratory endpoints were assessed.
IMPALA Open-Label Period
A total of 128 patients completed the open-label period of IMPALA. Sixty-eight patients enrolled in the open-label period through week 48. The other 60 patients were enrolled under an early protocol version of the study through week 72.
During the double-blind period, a dose frequency dependency was observed in most endpoints with continuous administration of Molgradex showing higher efficacy than placebo and continuous administration appearing to result in higher efficacy than once daily dosing every other week. Open-label results presented below focus on the group that had received a once daily continuous dose (OD) of Molgradex during the double-blind period versus those that had received once daily placebo (PBO) during the double-blind period―both of which received once daily dosing every other week during the open-label period.
Improvements in Two Independent Measures of Gas Exchange
In addition to (A-a)DO2, diffusing capacity of the lungs for carbon monoxide (DLCO) is another way to assess gas exchange. As with (A-a)DO2, DLCO is impaired in patients with aPAP and was assessed as an additional secondary endpoint in the study.
Patients who had been in the OD treatment group during the double-blind period:
Improvement from baseline was maintained or progressively increased in these patients during the open-label period of the study. The average (A-a)DO2 improvement in the double-blind period was -12.1 mmHg at week 24, compared to an average improvement from baseline in the open-label period of -12.2 mmHg and -18.8 mmHg at weeks 48 and 72, respectively. (See Figure 1.) The mean absolute improvement in DLCO during the double-blind period was 11.6% predicted at week 24, compared to a mean absolute improvement from baseline in the open-label period of 13.7% and 19.8% predicted at weeks 48 and 72, respectively.
Patients who had been in the PBO group during the double-blind period:
PBO patients that transitioned to active drug in the open-label period showed similar average improvements to those who had been in the double-blind OD group. The average (A-a)DO2 improvement in the double-blind period was -8.8 mmHg at week 24, compared to an average improvement from baseline in the open-label period of -14.6 mmHg and -17.0 mmHg at weeks 48 and 72, respectively. (See Figure 1.) The mean absolute improvement in DLCO during the double-blind period was 3.9% predicted at week 24, compared to a mean absolute improvement from baseline in the open-label period of 14.2% and 18.0% predicted at weeks 48 and 72, respectively.
Improvements in SGRQ
Patients who had been in the OD treatment group during the double-blind period:
Changes from baseline in the SGRQ were maintained and further increased with longer exposure to Molgradex. The average improvement during the double-blind period was -12.3 points at week 24, compared to an average improvement from baseline in the open-label period of -13.1 and -17.4 points at weeks 48 and 72, respectively. (See Figure 2.)
Patients who had been in the PBO group during the double-blind period:
At the end of the open-label period, PBO patients that transitioned to active drug showed similar average improvements in the SGRQ to those who received OD in the double-blind period. The average improvement during the double-blind period was -4.7 points at week 24, compared to an average improvement from baseline in the open-label period of -14.7 and -14.9 points at weeks 48 and 72, respectively. (See Figure 2.)
Improvements in 6MWD
Patients who had been in the OD treatment group during the double-blind period:
The average improvement during the double-blind period was 39.6 meters at week 24, compared to an average improvement from baseline in the open-label period of 48.9 meters and 71.7 meters at weeks 48 and 72, respectively.
Patients who had been in the PBO group during the double-blind period:
The average improvement during the double-blind period was 6.0 meters at week 24, compared to an average improvement from baseline in the open-label period of 40.4 meters and 29.9 meters at weeks 48 and 72, respectively.
Improvements in WLL
During the double-blind period of the study, 33 whole lung lavage procedures were required, including 9 in the continuous group compared to 17 in the placebo group. During the open-label period of the study only 5 whole lung lavage procedures were conducted. The pre-study rate of WLL was 0.8 procedures per patient year, compared to 0.055 procedures per patient year during the open-label period of the study. (See Figure 3.)
The Company intends to present more comprehensive data from the IMPALA study at an upcoming scientific conference and/or submit it for consideration in a peer-reviewed journal.
Molgradex for NTM Lung Infection
The Company today announced top line microbiology results from OPTIMA, a Phase 2a, exploratory, open-label, non-controlled clinical study evaluating Molgradex for the treatment of NTM lung infection in patients not affected by cystic fibrosis (CF). The results, found below, will be discussed on today’s conference call/webcast.
OPTIMA Study Design
The OPTIMA study, comprised of a 48-week treatment period and a 12-week follow-up period, enrolled patients with either Mycobacterium avium complex (MAC) infection, or the more difficult-to-treat Mycobacterium abscessus (MABSC) infection. Prior to enrolling, all patients were diagnosed with bronchiectasis, with all except one having radiological evidence of nodular multifocal and/or cavitary bronchiectasis. Two treatment groups of patients were recruited into the study, both of which received Molgradex 300 µg administered once daily. Treatment group one consisted of patients who remained sputum culture positive while currently on a multidrug NTM guideline-based anti-mycobacterial regimen, which had been ongoing for at least six-months prior to the baseline visit. Treatment group two consisted of patients who remained sputum culture positive, but either stopped a multidrug NTM guideline-based anti-mycobacterial regimen at least 28 days prior to screening due to lack of response or intolerance, or never started such treatment.
Summary of Efficacy and Safety Results
Thirty-two patients were treated in the study, of which 24 had MAC infection and 8 had MABSC infection. Of the patients with MAC infection, 11 were in treatment group one and 13 were in treatment group two. Of the patients with MABSC infection, 3 were in treatment group one and 5 were in treatment group two.
Results from the Intention-to-Treat (ITT) population showed that 5 out of 24 patients (21%) with MAC infection achieved a sputum culture conversion, defined as at least three consecutive sputum samples without growth of nontuberculous mycobacteria during the treatment period. Two of these patients, one from each treatment group, remained culture negative through the 12-week follow-up period. Sputum culture conversions were not observed in patients with MABSC infection.
Among the 32 treated patients in the safety population, 14 experienced serious adverse events (SAEs), one of which was considered potentially treatment related. The most common SAE was infective exacerbation of bronchiectasis. Three patients died during the study, with all deaths unlikely related to the study treatment.
The Company will continue to assess data from the OPTIMA study to better understand the clinical outcomes seen in some patients. Next steps for the NTM program will be determined once results from ENCORE, an ongoing Phase 2a, open-label, non-controlled study evaluating Molgradex for the treatment of NTM in patients living with CF, are available.
The Company intends to submit the full data set from OPTIMA for consideration at an upcoming scientific conference.
AeroVanc
Enrollment continues in AVAIL, a pivotal Phase 3 clinical study of AeroVanc for the treatment of persistent methicillin-resistant Staphylococcus aureus (MRSA) lung infection in CF. Total target enrollment is 200 patients. The adult population is fully enrolled, and the Company has enrolled 133 patients out of a target of 150 in the primary analysis population (younger patients between 6-21 years of age). The Company will continue enrollment until Q2 2020 and anticipates enrolling a total of ~140 younger patients. Top line results are expected in early 2021.
Fourth Quarter Financial Results (Unaudited)
Savara's net loss for the fourth quarter of 2019 was
General and administrative expenses for the fourth quarter of 2019 and 2018 were
As of
Fiscal Year 2019 Financial Results
The Company’s net loss for the year ended
Research and development expenses increased by
General and administrative expenses increased by
During the year ended
Conference Call/Webcast
Savara management will host a conference call/webcast at
Approximately one hour after the call, a telephone replay will be available and will remain available through
About IMPALA
The IMPALA clinical study was a randomized, double-blind, placebo-controlled, Phase 3 clinical study of Molgradex in 138 patients with aPAP. Patients were randomized to receive treatment for 24 weeks in one of three arms: 1) Molgradex 300 µg administered once daily continuously over 24 weeks, 2) Molgradex 300 µg, and matching placebo, each administered once daily every other week, or 3) inhaled placebo administered once daily continuously over 24 weeks. At the end of the 24-week double-blind period, all treatment arms rolled into a 24-week open-label follow-up period and received Molgradex 300 µg administered once daily every other week. The primary endpoint of the study was change from baseline in alveolar-arterial oxygen gradient, or (A-a)DO2, a commonly used measure of oxygenation impairment, to week 24. In addition, three key secondary endpoints―St. George’s Respiratory Questionnaire (SGRQ), six-minute walk distance (6MWD), and time to requirement for whole lung lavage (WLL)―along with multiple other secondary and exploratory endpoints were assessed to determine improvement in the disease pathology and pathophysiology, clinical symptoms, and function.
About OPTIMA
The OPTIMA clinical study was an exploratory, open-label, non-controlled, multi-center, Phase 2a clinical study of Molgradex in 32 patients with persistent pulmonary NTM lung infection. OPTIMA enrolled patients with chronic Mycobacterium avium complex (MAC) infection or Mycobacterium abscessus (MABSC) infection, with all patients having either antibiotic refractory infection or intolerance to standard NTM antibiotics. Patients with cystic fibrosis were not enrolled. The study comprised a 48-week treatment period and a 12-week follow-up period. Two groups of patients were recruited into the OPTIMA study. Treatment group one consisted of patients who remained sputum culture positive while currently on a multidrug NTM guideline-based anti-mycobacterial regimen, which had been ongoing for at least six months prior to the baseline visit. Treatment group two consisted of patients who remained sputum culture positive, but either stopped a multidrug NTM guideline-based anti-mycobacterial regimen at least 28 days prior to screening due to lack of response or intolerance, or never started such treatment.
The primary endpoint in the study was sputum culture conversion, defined as at least three consecutive sputum samples without growth of nontuberculous mycobacteria. Secondary endpoints included: (i) the number of patients with sputum smear conversion to negative, defined as at least three consecutive negative acid-fast bacilli (AFB) stained sputum smears on microscopy among patients who were smear positive at baseline, (ii) the number of patients with durable sputum culture conversion, defined as sputum culture conversion at or before week 48 and culture still negative for growth of nontuberculous mycobacteria at 12-week follow-up, (iii) the number of patients with durable sputum smear conversion, defined as sputum smear conversion at or before week 48 and AFB stained smear still negative for nontuberculous mycobacteria at 12-week follow-up among patients who were smear positive at baseline, and (iv) other microbiological indicators, exercise capacities, and patient reported outcomes.
About AVAIL
AVAIL is a randomized, double-blind, placebo-controlled, Phase 3 study of vancomycin hydrochloride inhalation powder (AeroVanc) in people living with cystic fibrosis who have methicillin-resistant Staphylococcus aureus (MRSA) lung infection. Target enrollment is 200 patients. During Period 1 of the study, patients are randomly assigned in a blinded 1:1 fashion to receive either AeroVanc (30 mg) twice daily, or placebo, by inhalation for 24 weeks or 3 dosing cycles. A dosing cycle is defined as 28 days of treatment followed by 28 days of observation. During Period 2 of the study, patients receive open-label AeroVanc (30 mg) twice daily for an additional 24 weeks or 3 dosing cycles, to evaluate the long-term safety of AeroVanc.
The primary endpoint is the mean absolute change in FEV1 percent predicted from baseline, which will be analyzed sequentially at week 4 (the end of cycle 1), at week 12 (the end of cycle 2) and at week 20 (the end of cycle 3). Analysis of the primary endpoint will be based on patients between 6-21 years of age. Secondary efficacy endpoints include: (i) time-to-use of another antibiotic medication for pulmonary infection, (ii) the number of successful FEV1-response cycles a patient achieves over Period 1 (weeks 4, 12, and 20), (iii) relative change from baseline in FEV1 percent predicted at weeks 4 and 20, (iv) change from baseline CF Questionnaire-Revised scores at weeks 4 and 20 and (v) change from Baseline in CF Respiratory Symptom Diary-Chronic Respiratory Symptom scores at weeks 4 and 20.
About Savara
Savara is an orphan lung disease company. Savara’s pipeline comprises Molgradex, an inhaled granulocyte-macrophage colony-stimulating factor (GM-CSF) in Phase 3 development for autoimmune pulmonary alveolar proteinosis (aPAP), in Phase 2a development for nontuberculous mycobacterial (NTM) lung infection in both non-cystic fibrosis (CF) and CF-affected individuals with chronic NTM lung infection, and AeroVanc, an inhaled vancomycin in Phase 3 development for persistent methicillin-resistant Staphylococcus aureus (MRSA) lung infection in CF. Savara’s strategy involves expanding its pipeline of potentially best-in-class products through indication expansion, strategic development partnerships, and product acquisitions with the goal of becoming a leading company in its field. Savara’s management team has significant experience in orphan drug development and pulmonary medicine, identifying unmet needs, developing and acquiring new product candidates, and effectively advancing them to approvals and commercialization. More information can be found at www.savarapharma.com. (Twitter: @SavaraPharma, LinkedIn: www.linkedin.com/company/savara-pharmaceuticals/).
Forward-Looking Statements
Savara cautions you that statements in this press release that are not a description of historical fact are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words referencing future events or circumstances such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, statements regarding our strong belief that Molgradex could improve outcomes for patients with aPAP, that we continue conversations with the FDA, that our highest priority is to get clarity from the agency regarding the design and endpoints of a second Phase 3 study in aPAP, that the Company intends to present more comprehensive data from the IMPALA study at an upcoming scientific conference and/or submit it for consideration in a peer-reviewed journal, that the Company will continue to assess data from the OPTIMA study to better understand the clinical outcomes seen in some patients, that next steps for the NTM program will be determined once results from ENCORE are available, that the Company intends to submit the full data set from OPTIMA for consideration at an upcoming scientific conference, that the Company will continue AVAIL enrollment until Q2 2020, anticipates enrolling a total of ~140 younger patients and top line results are expected in early 2021, statements regarding the planned discussions on today’s conference call/webcast, that the outstanding short- and long-term debt will continue to be used to bolster operations and advance the development of drug candidates and our strategy. Savara may not actually achieve any of the matters referred to in such forward-looking statements, and you should not place undue reliance on these forward-looking statements. These forward-looking statements are based upon Savara’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the outcome of our ongoing discussions with the FDA, risks and uncertainties associated with the outcome of our ongoing and planned clinical trials for our product candidates, the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources for Savara’s operations and to conduct or continue planned clinical development programs, the ability to obtain the necessary patient enrollment for our product candidates in a timely manner, the ability to successfully identify product acquisition candidates, the ability to successfully develop our product candidates, the risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates such as Molgradex and AeroVanc that are safe and effective for use as human therapeutics, and the timing and ability of Savara to raise additional equity capital as needed to fund continued operations. All forward-looking statements are expressly qualified in their entirety by these cautionary statements. For a detailed description of our risks and uncertainties, you are encouraged to review our documents filed with the
|
|
||||||||||||||||
|
Consolidated Statements of Operations and Comprehensive Loss |
||||||||||||||||
|
(in thousands, except for share and per share amounts) |
||||||||||||||||
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
Three months ended |
|
Years ended |
||||||||||||
|
|
|
|
|
|
||||||||||||
|
|
|
(Unaudited) |
|
|
||||||||||||
|
2019 |
|
2018 |
|
2019 |
|
2018 |
||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development |
$ |
8,723 |
|
$ |
9,857 |
|
$ |
38,781 |
|
$ |
37,173 |
|
||||
| General and administration |
|
3,296 |
|
|
3,252 |
|
|
13,081 |
|
|
10,654 |
|
||||
| Impairment of acquired IPR&D |
|
- |
|
|
- |
|
|
- |
|
|
21,692 |
|
||||
| Impairment of goodwill |
|
19,432 |
|
|
- |
|
|
26,852 |
|
|
- |
|
||||
| Depreciation and amortization |
|
58 |
|
|
139 |
|
|
311 |
|
|
526 |
|
||||
| Total operating expenses |
|
31,509 |
|
|
13,248 |
|
|
79,025 |
|
|
70,045 |
|
||||
| Loss from operations |
$ |
(31,509 |
) |
$ |
(13,248 |
) |
$ |
(79,025 |
) |
$ |
(70,045 |
) |
||||
| Other income, net |
|
(210 |
) |
|
232 |
|
|
852 |
|
|
1,472 |
|
||||
| Loss before income taxes |
$ |
(31,719 |
) |
$ |
(13,016 |
) |
$ |
(78,173 |
) |
$ |
(68,573 |
) |
||||
| Income tax benefit |
|
- |
|
|
2,502 |
|
|
- |
|
|
7,057 |
|
||||
| Net loss |
$ |
(31,719 |
) |
$ |
(10,514 |
) |
$ |
(78,173 |
) |
$ |
(61,516 |
) |
||||
| Net loss per share - basic and diluted |
$ |
(0.72 |
) |
$ |
(0.29 |
) |
$ |
(1.95 |
) |
$ |
(1.85 |
) |
||||
| Weighted average shares - basic and diluted |
|
43,822,367 |
|
|
35,903,728 |
|
|
40,027,758 |
|
|
33,300,704 |
|
||||
| Other comprehensive expense |
|
219 |
|
|
(164 |
) |
|
(217 |
) |
|
(758 |
) |
||||
| Total comprehensive loss |
$ |
(31,500 |
) |
$ |
(10,678 |
) |
$ |
(78,390 |
) |
$ |
(62,274 |
) |
||||
| Consolidated Balance Sheet data | ||||||
| (in thousands) | ||||||
|
|
|
|
||||
|
2019 |
|
2018 |
||||
| Cash, cash equivalents, and short-term investments |
$ |
121,761 |
$ |
110,830 |
||
| Working capital |
|
113,187 |
|
106,090 |
||
| Total assets |
|
136,203 |
|
152,287 |
||
| Total liabilities |
|
34,505 |
|
44,068 |
||
| Stockholders' equity |
|
101,698 |
|
108,219 |
||
View source version on businesswire.com: https://www.businesswire.com/news/home/20200312005455/en/
(512) 851-1366
Source:
