

Release
Savara Announces Results of IMPALA, a Phase 3 Study of Molgradex for the Treatment of Autoimmune Alveolar Pulmonary Proteinosis (aPAP)
- The study did not meet its primary endpoint of alveolar-arterial oxygen gradient (A-aDO2)
- The study showed statistically significant improvement in the St. George’s Respiratory Questionnaire (SGRQ), a key secondary endpoint
- Adverse event frequencies were similar between the treatment arms and placebo
- Company plans to meet with regulatory authorities in the coming months to discuss results from the study and a path forward
- Management to host conference call today at
5:30 p.m. ET
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190612005880/en/
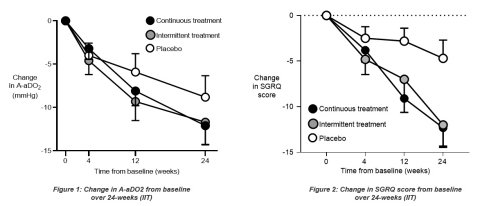
Figure 1 and Figure 2
Primary Endpoint in the Intention-to-Treat (ITT) Population
An average A-aDO2 improvement of 12.1 mmHg was observed in the continuous dosing arm, compared to an average A-aDO2 improvement of 8.8 mmHg in the placebo arm. With an estimated 4.6 mmHg treatment difference (p=0.17), the study did not meet its primary endpoint.
Secondary Endpoints in the ITT Population
An average improvement of 12.3 points in the SGRQ (a patient-reported outcomes/quality-of-life measure) was observed in the continuous dosing arm compared to an average improvement of 4.7 points in the placebo arm. The estimated treatment difference was 7.6 points (p=0.01) which was statistically significant and approximately two times the generally accepted clinically meaningful difference of four points for this measure.
The other two key secondary endpoints, the six-minute walk distance (6MWD) and requirement for whole lung lavage (WLL), were numerically in favor of the continuous dosing arm, but the differences to placebo were not statistically significant. Patients in the continuous dosing arm showed a mean improvement of 39.6 meters in the 6MWD, while the intermittent and placebo arms showed improvements of 11.3 meters and 6.0 meters, respectively. Four patients in each of the active arms, and six patients in the placebo arm underwent a WLL during the treatment period. Given that some patients received more than one WLL, the total number of WLLs observed in the continuous dosing arm was 9, with 7 observed in the intermittent dosing arm and 17 in the placebo arm.
In addition to the A-aDO2, the diffusing capacity of the lungs for carbon monoxide (DLCO) was assessed as a secondary endpoint to evaluate the efficacy of Molgradex on gas transfer. Patients in the continuous dosing arm showed a mean improvement of 11.6% predicted in DLCO, whereas the intermittent dosing and placebo arms showed a 7.7% predicted and 3.9% predicted improvement, respectively. The estimated treatment difference of 7.9% predicted (p=0.007) between the continuous dosing arm and placebo was statistically significant.
“Taken together, the trends in reduction of A-aDO2,as
well as the improvement in the diffusion capacity, are very consistent
with reduction of the surfactant burden that causes the clinical
symptoms of aPAP,” said
Safety and Tolerability in the ITT Population
The number of treatment emergent adverse events (TEAEs), including serious adverse events, occurred with similar frequency and severity in the active arms compared to placebo. The number of subjects discontinuing due to TEAEs was as follows: continuous dosing arm; n=2, intermittent dosing arm; n=1, placebo arm; n=1.
The Company plans to meet with the
"Disappointingly, with the placebo effect stronger than anticipated, the
study did not meet its primary endpoint,” said
The Company intends to submit the full data set from IMPALA to a peer-reviewed scientific journal or to an upcoming medical meeting for consideration.
Conference Call and Webcast
The Company will host a conference call today at
Approximately one hour after the call, a replay of the webcast will be
available on Savara’s website for 30 days, and a telephone replay will
be available through
About the IMPALA Phase 3 Clinical Study
The IMPALA study is a randomized, double-blind, placebo-controlled study
designed to compare the efficacy and safety of Molgradex with placebo in
patients with aPAP. The study is being conducted in 18 countries
worldwide. Patients were randomized to receive treatment for 24 weeks in
one of three arms: 1) Molgradex 300 µg administered once daily
continuously over 24 weeks, 2) Molgradex 300 µg, and matching placebo,
administered once daily in 7-day intermittent cycles of each, or 3)
inhaled placebo administered once daily continuously over 24 weeks. At
the end of the 24-week double-blind period, all treatment arms roll into
a 24-week open-label follow-on period and receive Molgradex 300 ug
administered daily in 7-day intermittent cycles. The primary endpoint in
the study is the absolute change from baseline in A-aDO2, a
measure of the patient’s oxygenation status, following 24 weeks of
treatment. In addition, the
About Savara
Savara is an orphan lung disease company. Savara’s pipeline comprises Molgradex, an inhaled granulocyte-macrophage colony-stimulating factor (GM-CSF) in Phase 3 development for autoimmune pulmonary alveolar proteinosis (aPAP), in Phase 2a development for nontuberculous mycobacterial (NTM) lung infection in both non-cystic fibrosis (CF) and CF-affected individuals with chronic NTM lung infection; and AeroVanc, a Phase 3-stage inhaled vancomycin for treatment of persistent methicillin-resistant Staphylococcus aureus (MRSA) lung infection in CF. Savara’s strategy involves expanding its pipeline of potentially best-in-class products through indication expansion, strategic development partnerships and product acquisitions, with the goal of becoming a leading company in its field. The most recent acquisition is aerosolized amikacin/fosfomycin, a Phase 2-ready combination antibiotic for which the initial indication will focus on non-CF bronchiectasis patients with chronic lung infection and frequent exacerbations. Savara’s management team has significant experience in orphan drug development and pulmonary medicine, identifying unmet needs, developing and acquiring new product candidates, and effectively advancing them to approvals and commercialization. More information can be found at www.savarapharma.com. (Twitter: @SavaraPharma, LinkedIn: www.linkedin.com/company/savara-pharmaceuticals/)
Forward-Looking Statements
Savara cautions you that statements in this press release that are not a
description of historical fact are forward-looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995.
Forward-looking statements may be identified by the use of words
referencing future events or circumstances such as “expect,” “intend,”
“plan,” “anticipate,” “believe,” and “will,” among others. Such
statements include, but are not limited to, Dr. Trapnell’s statements
regarding trends in reduction of A-aDO2 as well as the
improvement in the diffusion capacity being very consistent with
reduction of the surfactant burden that causes the clinical symptoms of
aPAP, the impressive improvement in quality of life, as measured by the
SGRQ, suggest that Molgradex not only improved objective measures of
oxygenation, but also had a clinically meaningful therapeutic effect and
the belief that Molgradex can become an important pharmacological
treatment option for patients with aPAP, statements related to Savara’s
plans to meet with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20190612005880/en/
Source:
Savara Inc. IR & PR
Anne Erickson (anne.erickson@savarapharma.com)
(512)
851-1366
For IR: Solebury Trout
Gitanjali Jain Ogawa (Gogawa@troutgroup.com)
(646)
378-2949
