

Filing
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) February 12, 2018
SAVARA INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-32157 | 84-1318182 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
6836 Bee Cave Road, Building III, Suite 200
Austin, TX 78746
(Address of principal executive offices, including zip code)
(512) 961-1891
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
Attached hereto as Exhibit 99.1 is an updated corporate presentation for Savara Inc. (“Savara,” “we” or “our”).
Savara may announce material information about its finances, product candidates, clinical trials and other matters to its investors using its investor relations website (http://savarapharma.com/investors), SEC filings, press releases, public conference calls and webcasts. We use these channels, as well as social media, to communicate with our stockholders and the public about our company and other issues. It is possible that the information we post on our website and social media could be deemed to be material information. Therefore, we encourage investors, the media, and others interested in our company to review the information we post on our investor relations website (referenced above) and any social media channels listed on our website from time to time.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description | |
| 99.1 | Savara Corporate Presentation | |
-2-
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: February 12, 2018 | SAVARA INC. a Delaware corporation | |||||
| By: | /s/ Dave Lowrance | |||||
| Dave Lowrance Chief Financial Officer | ||||||
-3-

Savara Corporate presentation (NASDAQ: SVRA) February 2018 Exhibit 99.1

Safe Harbor Statement Savara Inc. (“Savara” or the “Company”) cautions you that statements in this presentation that are not a description of historical fact are forward-looking statements which may be identified by the use of words such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, statements regarding the sufficiency of our resources to fund the advancement of any of our development programs or the completion of any clinical trial; the nature, strategy and focus of our organization; the safety, efficacy and projected development timeline and commercial potential of any of our product candidates; the potential health benefits of our product candidates; our anticipated corporate milestones and the market size or potential for any of our products. Savara may not actually achieve any of its plans or product development goals in a timely manner, if at all, or otherwise carry out its current intentions or meet the expectations or projections disclosed in its forward-looking statements, and you should not place undue reliance on these forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon Savara's current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources for our operations and to conduct or continue planned clinical development programs, the timing and ability of Savara to raise additional equity capital as needed to fund continued operations; the ability to successfully conduct clinical trials for our product candidates; the ability to successfully develop any of Savara's product candidates, and the risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates that are safe and effective for use as human therapeutics. The risks and uncertainties facing Savara are described more fully in Savara's filings with the Securities and Exchange Commission including our filings on Form 8-K, our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, and our Quarterly Report on Form 10-Q for the quarter ended September 30, 2017. You are cautioned not to place undue reliance on our forward-looking statements, which speak only as of the date on which they were made. Savara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.

Investment Highlights Pipeline Multiple late-stage assets addressing rare respiratory diseases Known treatment concepts – clinical risk reduction Over $1B peak sales potential* of two lead assets Near-term data readout and milestones Company World-class team with track record of developing respiratory products September 30, 2017: Cash of ~$53.3 million October 2017: ~$50 million public offering Analyst coverage: Jefferies, JMP, Canaccord, Ladenburg, ROTH *Savara estimate based on MME 2017 survey

Late-stage pipeline Pre-Clinical Molgradex* AeroVanc Aironite** Phase 1 Phase 2 Phase 3 * Serendex acquisition in July 2016 ** Mast acquisition in April 2017 Molgradex Pulmonary Alveolar Proteinosis (PAP) MRSA in Cystic Fibrosis (CF) Diastolic Heart Failure (HFpEF) Nontuberculous Mycobacteria (NTM) IMPALA enrollment complete Q3 2018 AVAIL enrollment complete Q1 2019 INDIE study top line results expected 1H 2018 Phase 2a study expected to begin Q1 2018

Molgradex Inhaled GM-CSF for Autoimmune Pulmonary Alveolar Proteinosis (aPAP)

PAP: excess of surfactant in the Lungs Anti-GM-CSF antibodies cause accumulation of surfactant in the alveoli Currently treated by whole lung lavage (WLL) Decreased oxygen delivery Hypoxia and shortness of breath US prevalence of ~2,500 patients* Typical onset 30-50 ys Mechanism of disease well understood *Trapnell BC, et. al. Am J Respir Crit Care Med. 2014

Inhaled GM-CSF Promising in academic studies* aPAP patient before inhaled GM-CSF After 6 months of inhaled GM-CSF Improvement in alveolar to arterial oxygen gradient ((A-a)DO2) Published experience from treatment of more than 80 aPAP patients suggests potential for significant impact on oxygenation and clinical symptoms * Tazawa R, et al. (2010) Inhaled GM-CSF as Therapy for Pulmonary Alveolar Proteinosis. Am J Resp Crit Care Med * Wylam ME, et al. (2006). Aerosol GM-CSF for pulmonary alveolar proteinosis. Eur Respir * Papiris SA, et al. (2014). Longterm inhaled GM-CSF in aPAP. Clin Drug Investig

Molgradex: First Inhaled GM-CSF Inhalation solution of rhGM-CSF eFlow® Nebulizer (PARI Pharma)* Proprietary cell bank for GM-CSF manufacturing Orphan exclusivity (7 yrs US + 10 yrs EU) *investigational eFlow Nebulizer (PARI Pharma)

Phase 1 – consistent WBC response to each dose Safety in healthy volunteers comparable to placebo Potent dose-dependent pharmacodynamic effect

Design OF Ongoing IMPALA Phase 3 Study Primary Endpoint Change from baseline in ((A-a)DO2) FDA to evaluate efficacy based on 3 alternative secondary endpoints adjusted for multiplicity Secondary Endpoints 6 min walk distance St. George’s respiratory questionnaire Requirement for/time to WLL Screening BL n=45, 300µg, daily dosing Period 1- Double-blind W 8 W 12 W 24 W 16 W 20 W 4 Period 2- Follow-up* n=45, 300µg, daily dosing every other week n=45, placebo W 48 *Open-label Molgradex, every other week dosing

Molgradex Inhaled GM-CSF for Nontuberculous Mycobacterial (NTM) Lung Infection
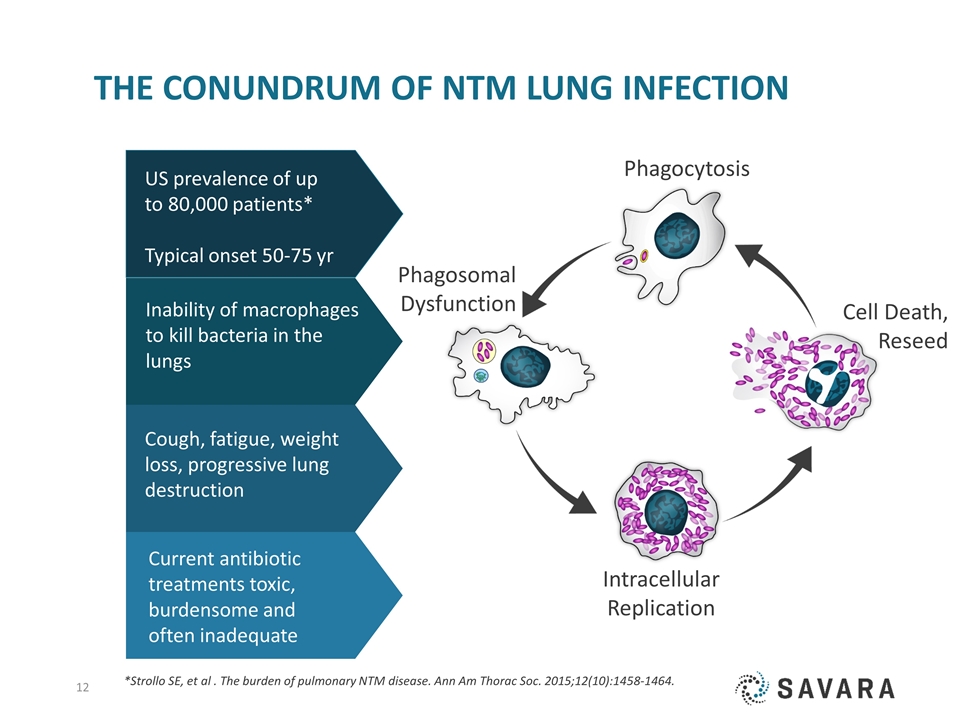
The conundrum of NTM Lung INFECTiON Inability of macrophages to kill bacteria in the lungs Current antibiotic treatments toxic, burdensome and often inadequate Cough, fatigue, weight loss, progressive lung destruction US prevalence of up to 80,000 patients* Typical onset 50-75 yr Cell Death, Reseed Phagocytosis Phagosomal Dysfunction Intracellular Replication *Strollo SE, et al . The burden of pulmonary NTM disease. Ann Am Thorac Soc. 2015;12(10):1458-1464.

emerging Scientific support of GM-CSF for NTM* Inhaled INF-γ eradicated pulmonary M. abscessus Animal studies support key role of GM-CSF in chronic NTM lung infection Systemic GM-CSF explored in systemic NTM infection Inhaled GM-CSF eradicated / reduced M. abscessus** Candidate for Orphan & QIDP status * deSilva T.I., et al., Journal of Infection (2007) 54 (e207-e210) * Hallstrand T.S., et al., European Respiratory Journal (2004) 24 (367-370) * Groote et al., Journal of Antimicrobial Chemotherapy (2014) 69 (1057-64) * Bermudez, et al., Journal of Infectious Diseases (1994) 169 (575–580) ** Scott et al., European Respiratory Journal (2018) (DOI: 10.1183/13993003.02127-2017)

inhaled GM-CSF promising in pulmonary NTM* High positive smear and culture for M. abscessus Multi-drug antibiotics unsuccessful Dire clinical condition Inhaled GM-CSF for >1.5 years (first 3 mo with abx) Smear and culture negative (incl. BAL) Considerable clinical improvement High positive smear and culture for M. abscessus No antibiotics attempted Clinical worsening Inhaled GM-CSF for >6 mo (no abx) Smear negative and culture to “one colony” Clinical improvement Case 1: 11-year old female Case 2: 28-year old male * Scott et al., European Respiratory Journal (2018) (DOI: 10.1183/13993003.02127-2017)

Molgradex Phase 2a OPEN-LABEL Study in NTM Primary Endpoint NTM sputum culture conversion to negative Secondary Endpoints NTM sputum smear conversion to negative Durability of NTM sputum conversion Reduction of NTM in sputum Change in 6 min walk distance Change in body weight Change in QoL and symptom scores Screening BL Group 1 – On antimycobacterial treatment Period 1- 300 µg Molgradex W 8 W 12 W 24 W 16 W 20 W 4 Period 2- Follow-up Group 2 – Not on antimycobacterial treatment W 12 12-weeks follow-up period Study expected to start in Q1 2018

AeroVanc Inhaled Vancomycin for MRSA in Cystic Fibrosis

HIGH UNMET NEED for INHALED MRSA Treatment Persistent lung infections managed with chronic inhaled antibiotics No approved inhaled MRSA antibiotic, emerging use of nebulized IV form of vancomycin MRSA infection associated with worse clinical outcomes CF Prevalence (US)* 30,000 patients, 26% MRSA infected Source: 2015 CFF Patient Registry Dasenbrook, et al. reprinted with permission, Copyright © (2010) JAMA *O’Sullivan BP, Freedman SD. Lancet 2009;373:1891–1904

AeroVanc: First Inhaled MRSA Antibiotic Grants from CFF & NIH Inhaled Dry Powder Vancomycin Drug Directly to Site of Infection Designated Fast Track by the FDA Manufacturing at Commercial Scale Orphan 7 yrs + QIDP 5 yrs (total 12 years exclusivity)

FEV1 Improvement Consistent with TOBI Data PP population, 32 mg dose cohort, < 21 years of age, n = 16, post hoc analysis Best FEV1 improvement in young patients Similar FEV1 response profile in prior TOBI studies Absolute change in FEV1 selected as Phase 3 primary endpoint Phase 3 to be powered and focused on children 6-21 years

AVAIL Phase 3 STUDY design (per FDA guidance) Screening Period 1- Double-blind Period 2- Open-label BL Placebo, n = 100 AeroVanc 32mg, n = 100 W 8 W 12 W 24 W 16 W 20 W 4 AeroVanc 32mg, n = ~150-170 W 48 FEV 1 improvement (relative change, number of response cycles) FEV 1 improvement at Week 4 and Week 20 (absolute change analyzed sequentially) Respiratory Symptoms Diary Time to use of another antibiotic for pulmonary infection Secondary Endpoints Primary Endpoint Primary analysis based on patients 6-21 years of age

FINANCIALS AND MILESTONES

Savara FINANCIAL OVERVIEW Public follow-on offerings completed in Q2 & Q3/2017 ~$100 million total gross proceeds Cash as of September 30, 2017 ~$53.3 million Debt facility $15 million Common stock issued and outstanding as of November 8, 2017 30,500,693 Fully diluted capitalization as of November 8, 2017 34,553,737 Runway Into 2020

KEY CLINICAL MILESTONES H1 ‘18 H2 ‘18 H1 ‘19 H2 ‘19 Molgradex IMPALA Phase 3 Top Line Results Aironite Phase 2 INDIE Study Top Line Results AeroVanc AVAIL Phase 3 Study Top Line Results Molgradex IMPALA Phase 3 Study Enrollment Completes Molgradex Phase 2a NTM Study Initiation AeroVanc AVAIL Phase 3 Study Enrollment Completes Molgradex Phase 2a NTM Study Enrollment Complete

